Equal Volumes of .2m Solutions of Lead
Click hereto get an answer to your question 20 mL of 02 M NaOH is added to 50 mL of 02 M acetic acid to give 70 mL of the solution. The rate of dissolving of the solid is A.

Ml Aggarwal Solutions For Class 10 Maths Chapter 17 Mensuration
Ksp for lead II iodide.
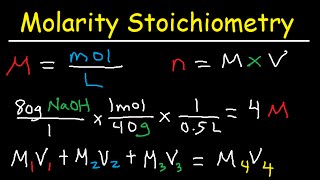
. We know n00500 mol and c200 moll. Which of the following is the correct net ionic equation for the reaction. Number of moles and V.
Equal to the rate of precipitation. The ionisation constant of acetic acid is 18 10-5. Equal volumes of 02M solutions of leadII nitrate and potassium bromide are combined to form leadII bromide as a yellow precipitate.
We know that cnV with c. V 00500 mol2moll 0025 l. Click hereto get an answer to your question If equal volumes of 1M KMnO4 and 1M K2Cr2O7 solutions are allowed to oxidize Fe2 in acidic medium.
If the volumes used were both 150ml what is the mass of the precipitate assuming the reaction goes to completion. Your solution has a concentration of 2 moll. Pb2aq2BraqPbBr 2 s KaqNO 3 aqKNO 3 aq Pb2aq2NO 3 aq2Kaq2Braq2Kaq2NO 3 aqPbBr 2 s PbNO 3 2.
Pb 2 aq 2 Br-aq-- PbBr 2 s K aq. A Pb 2 aq 2Br. Na 2 Cr 2 O 7 C.
Equal volumes of 02 M solutions of lead II nitrate and potassium bromide are combined to form lead 11 bromide as a yellow precipitate. A student prepares a 2M solution of copper sulphate. Ionic product of copper iodate.
Solve Study Textbooks Guides. Example 1 Will a precipitate form when equal volumes of 2M PbNO3 and 2M KI are mixed. We can use the inverse of this ratio to find how many moles of lead II nitrate we need for every 001 moles of potassium phosphate.
Answer 1 of 2. How many moles of copper sulphate are in the 100 mL solution. For dilute solutions it can be assumed that the volume of a diluted solution is equal to the sum of the volume of the original solution and the volume of solvent added V.
She adds 17 mL of the solution to a 100 mL volumetric flask and dilutes it up to a final volume of 100 mL with water. For cupric iodate Ksp 74 10-8. View example-1-njpg from CARRERTECHNOLOGY EDUCATION 79780600 at The Colony H S.
Pb² I PbI₂ 2006- Solid potassium chlorate is strongly heated. Na 2 CrO 4. Which of the following is the correct net ionic equation for the reaction.
Equal volumes of 02 M solutions of leadII nitrate and potassium bromide are combined to form leadII bromide as a yellow precipitate. Less than the rate of precipitation. What is the pH of this solution.
We are given the molarity and the volume of solution. The amount of iron oxidized will be. The only issue is that the volume is given in mL instead of L.
S-75-a Calculate the molarity of a solution made by adding 125 mL of water to 55 mL of a 200 M NaOH solution. 2005- Equal volumes of 01 M solutions of lead II nitrate and magnesium iodide are combined. We need 3 moles of lead II nitrate solution for every 2 moles of potassium phosphate solution so we have a ratio of 23.
Up to 24 cash back possible to redissolve this precipitate by adding a concentrated solution of A. Equal volumes of 02M solutions of leadII nitrate and potassium bromide are combined to form leadII bromide as a yellow precipitate. This issue can be fixed by using the following conversion factor.
Will it lead to precipitation of copper iodate. Click hereto get an answer to your question Equal volumes of 0002 M solutions of sodium iodate and cupric chlorate are mixed together. Aq PbBr2 s 2K.
Calculate the additional volume of 02 M NaOH required to make the pH of the solution 474. Equal volumes of 02M solutions of leadII nitrate and potassium bromide are combined to form leadII bromide as a yellow precipitate. 500 ml of 2M NaCl solution was mixed with 200 ml of 2 M NaCl solution.
Colorwhiteaaaaaaaaaaaaaaaaaaaaa1000mL 1L Therefore if we divide 50mL by 1000mL we will obtain a value of 005L. Aq NO3- aq KNO3 s C Pb 2 aq 2NO3- aq 2K aq 2Br. What is the correct net ionic equation for the reaction.
If equal volumes of 1 M K M n O 4 and 1 M K 2 C r 2 O 7 solutions are allowed to oxidize F e 2 in. If the concentrations of both solutions are equal to or less than 502 10 9 M then there will be no precipitation of iron sulphide. 001320015 moles lead II nitrate We know this is correct because 001001523.
When equal volumes of sodium iodate and cupric chlorate solutions are mixed together then the molar concentrations of both solutions are reduced to half ie 0001 MThen Now the solubility equilibrium for copper iodate can be written as. What is the final molar concentration of the copper sulphate. 4 Equal volumes of 02M solutions of leadII nitrate and potassium bromide are combined to form leadII bromide as a yellow precipitate.
Which of the following is the correct net ionic equation for the reaction. Transforming the equation will lead to Vnc. Equal volumes of 02M solutions of leadII nitrate and potassium bromide are combined to form leadII bromide as a yellow precipitate.
A beaker contains an unsaturated solution and some solid solute. Calculate the molarity of NaCl in final solution. Aq PbBra s B K.
After mixing the volume of the concentrations of each solution will be reduced to half ie. Let the maximum concentration of each solution be x molL.
200ml Of 0 2m Hcl Is Neutralised With 0 1m Naoh Then During Their Half Neutralization What Will Be The Molarity Of Hcl Quora

Ml Aggarwal Solutions For Class 10 Maths Chapter 17 Mensuration

Molarity Dilution Problems Solution Stoichiometry Grams Moles Liters Volume Calculations Chemistry Youtube

Methods For Preparing Buffers Video Khan Academy
What Is The Concentration Of Cl Ion In A Solution By Mixing 300 Ml 0 3 M Nacl And 200 Ml Of 0 4 Bacl2 Quora
A Strip Of Zinc Was Placed In A Beaker That Contained 120ml Of Copper Ii Nitrate Cu No3 2 Aq The Mass Of The Copper Produced Was 0 813g Find The Initial Concentration Of

Free Iv Fluid Guide And Cheat Sheet 2020 Update Iv Solutions Extracellular Fluid Critical Care Nursing

Ml Aggarwal Solutions For Class 10 Maths Chapter 17 Mensuration

Stoichiometry Of Precipitation Reactions And Remaining Ion Concentration

Ap Chemistry Unit 9 Buffers Titrations Ksp Quizizz

The Solubility Product Ksp Of Ca Oh 2 At 25 C Is 4 42 10 5 A 500 Ml Of Saturated Solution Of Ca Oh 2 Is Mixed With Equal Volume Of 0 4m Naoh How

Pdf Tide Dominated Deltas Responding To High Frequency Sea Level Changes Pre Messinian Rifian Corridor Morocco

Ap Chemistry Unit 9 Buffers Titrations Ksp Quizizz

How To Write The Net Ionic Equation For Kbr Pb No3 2 Kno3 Pbbr2 Youtube

200ml Of 0 2m Hcl Is Mixed With 300 M L Of 0 1m Naoh The Molar Heat Of Neutralization Of This Reaction Is 57 1kj The Increase In Temperature In C Of The System
What Is The Concentration Of Nitrate Ions If Equal Volumes Of 0 1m Agno3 And 0 1m Nacl Are Mixed Together Quora

Restaurant Server Resume Samples Server Resume Restaurant Resume Job Resume Examples
Comments
Post a Comment